ST. LOUIS — Wednesday is the first of two days of CDC meetings on potential Johnson and Johnson and Moderna booster shots.
The FDA has already said the booster doses are safe for Americans to take.
Now, it’s up to the CDC to give the stamp of approval before anyone can roll up their sleeves for another dose of these vaccines.
The FDA's advisory panel has recommended everyone who got a Johnson and Johnson COVID-19 shot get a second dose. The FDA didn’t issue age specifications for who qualifies for the booster shot with the J&J vaccine because it's been proven to have less efficacy than other shots.
They said the second dose of J&J should come at least two months after the first, since immunity fades over time.
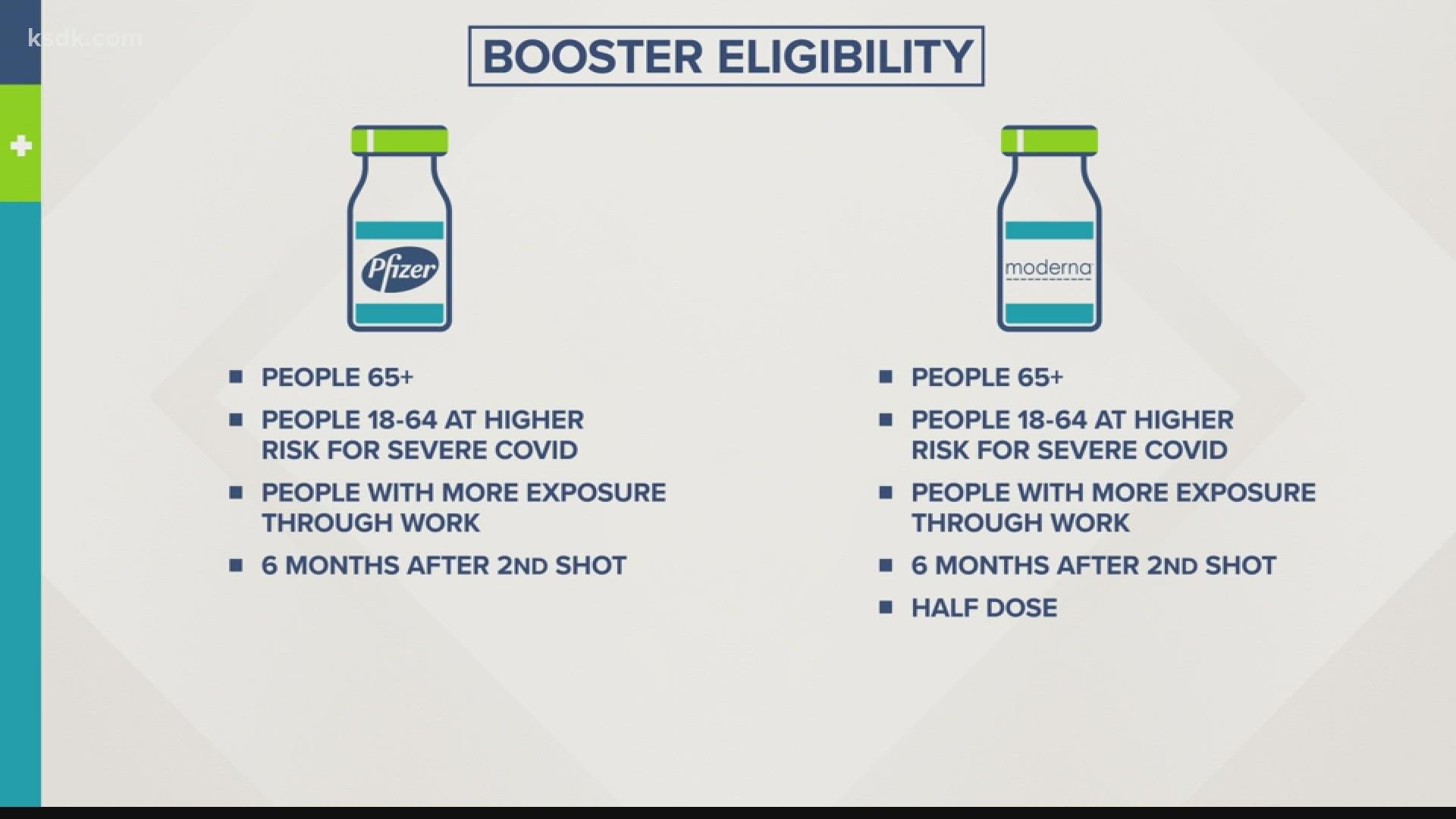
The FDA also recommends some of the people who have received a Moderna vaccine should also roll up their sleeves again.
The agency’s recommendations are similar to the ones that were made for Pfizer, which has already been approved by the CDC for booster shot use.
Pfizer and Moderna are both M-RNA vaccines and work similarly.
But, for booster shot approval, the companies are seeking slightly different requirements.
Both makers are saying boosters should be approved for people over 65 and adults with underlying health conditions. The companies recommend getting a booster dose six months after the second dose was administered.
But Moderna is only seeking approval for a half dose, not a full one like Pfizer.
The approval would mean more options for Americans as the FDA says it’s safe to mix vaccine brands when getting a booster shot.
“You don't have to worry about getting a Moderna shot if you got Moderna before or a Pfizer shot if you got Pfizer before, you really can get any of the vaccines as a booster and it will work just as well as the original,” said Dr. Ashish Jha from Brown University.