ST. LOUIS — Mercy and Mercy research are participating in the COVID-19 convalescent plasma program.
According to a press release from Mercy, in coordination with local blood donation programs it is participating in the U.S. Food and Drug Administration’s investigation into the use of plasma from recovered COVID-19 patients to treat COVID-19 patients meeting certain criteria.
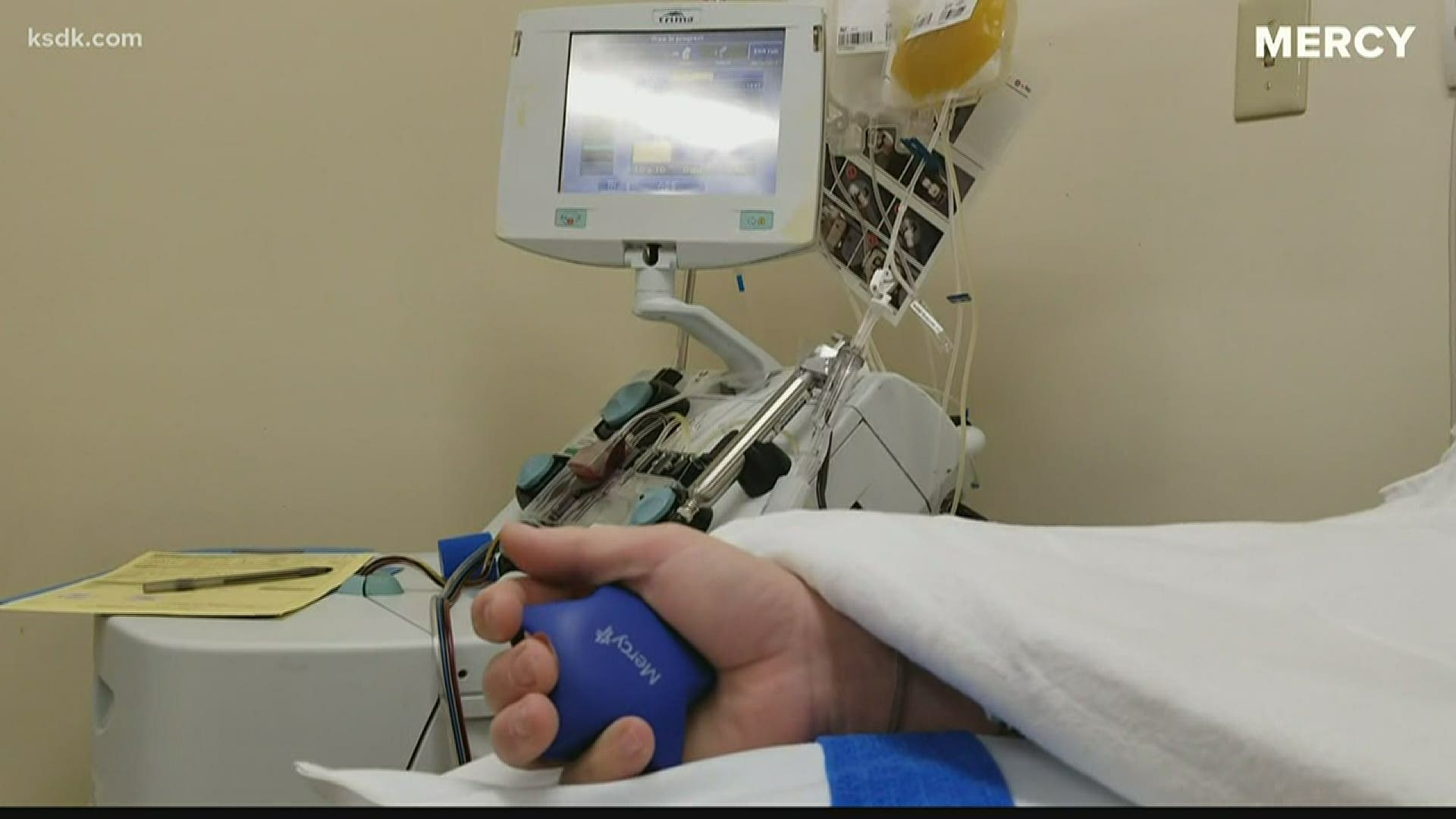
Mercy said it is in a unique position because of its own blood donor program. Mercy Hospital St. Louis began reaching out to potential donors April 4 and had its first eligible donation on April 6.
Donors must have a positive COVID-19 test result, be 28 days symptom-free and then test negative for COVID-19. Plasma from one donor can be used to treat up to three patients.
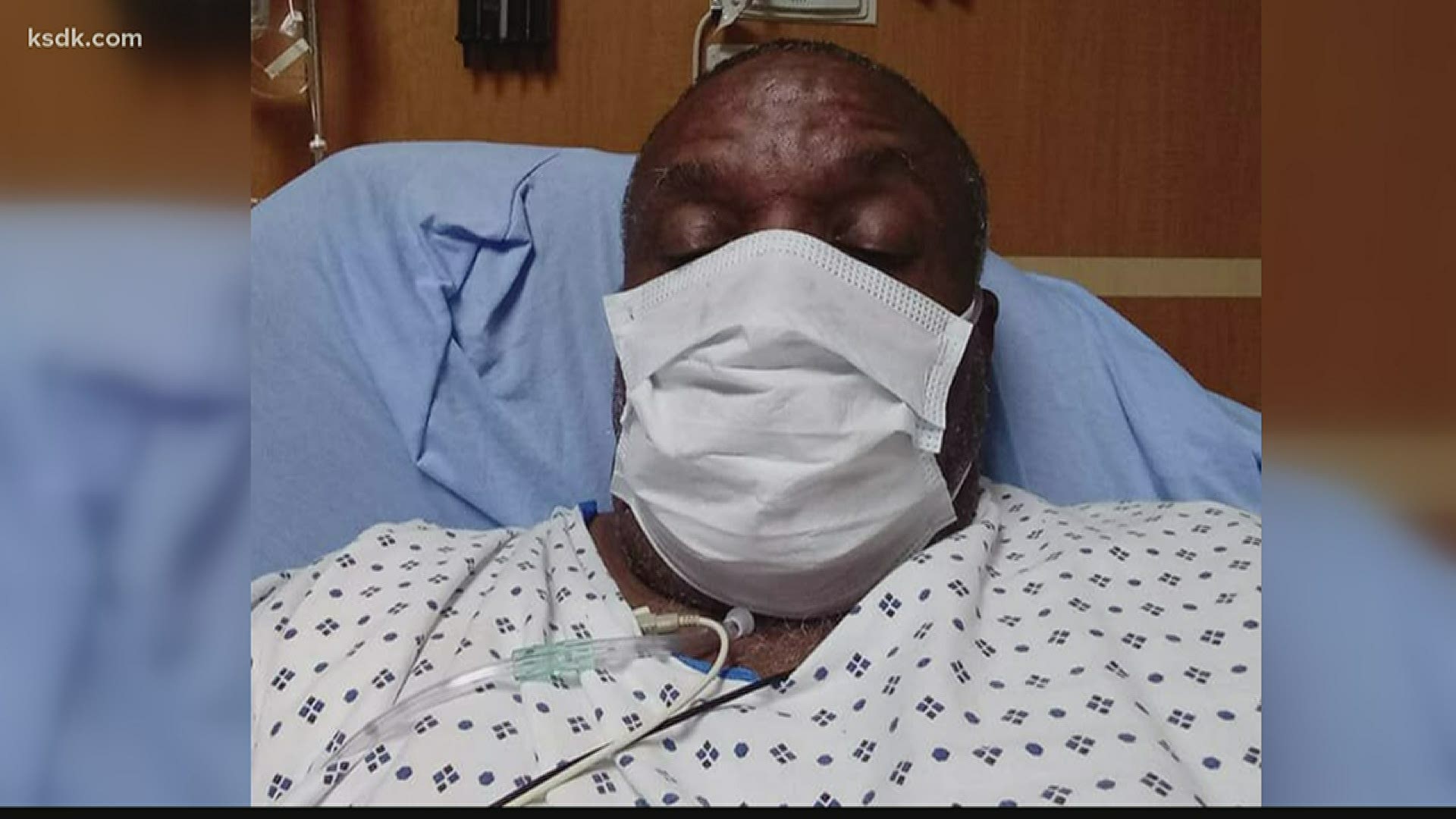
Walter Lamkin was the first recovered COVID-19 patient to donate his plasma at Mercy.
“When I got the call, I immediately said ‘Yes!’” said Walter Lamkin, who recently recovered from COVID-19 and donated his plasma. “I want to make something good out of a bad situation and hope to make more people aware of how easy the process is.”
The FDA said convalescent plasma that contains antibodies to SARS-CoV-2, the virus that causes COVID-19, might be effective in fighting the virus. It has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic.
“Our team is excited to participate and contribute to the possible treatment of COVID-19,” said Dr. Emily Schindler, medical director of Mercy Blood Donor Services in St. Louis. “The first donor was here quickly and just as excited to do his part to help others. We look forward to providing this option to appropriate COVID-19 patients across our health system.”
Mercy said convalescent plasma has not yet been shown to be effective specifically in treating COVID-19. The FDA’s COVID emergency investigational new drug and expanded access programs allow providers at participating hospitals to treat patients with or at risk of severe/life-threatening illness. Clinical trials and data-driven research studying convalescent plasma and other possible COVID treatments are also underway at research centers across the country, including through Mercy Research.
“As the nation manages this pandemic, Mercy is pleased to be at the cutting edge, offering a convalescent plasma option for our patients. With our own blood donor center in St. Louis and our strong relationships with community blood bank partners, Mercy was well-positioned to make the FDA program a reality,” said Dr. Fred McQueary, Mercy chief clinical officer and executive vice president. “Currently, we are actively obtaining convalescent plasma donations from our recovered patients, and we have already transfused our first three patients/recipients.”
Once collected, convalescent plasma will be distributed to hospitals treating COVID-19 patients. Treating physicians identify appropriate recipients, and patients must consent to the treatment. After the patient is transfused, caregivers must track and, as required, report data such as improvements or reactions.
The various agencies are contacting eligible donors. However, if you haven’t been contacted and have a positive COVID-19 test, please reach out.
- St. Louis metro: Mercy Blood Donor Services, Mississippi Valley Regional Blood Center and the American Red Cross
- Springfield, Missouri and Springdale, Arkansas: Community Blood Center of the Ozarks
- Oklahoma: Oklahoma Blood Institute
- Most of Arkansas: Arkansas Blood Institute
OTHER STORIES